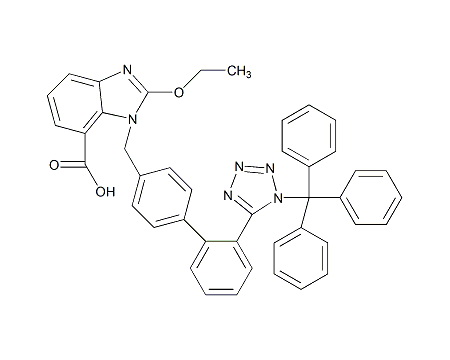
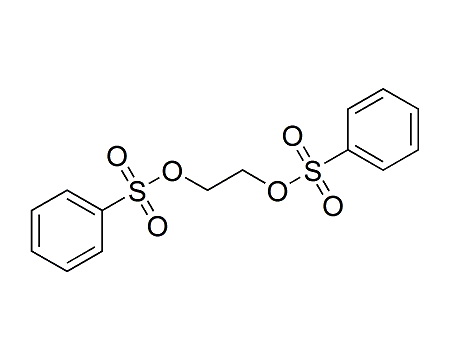
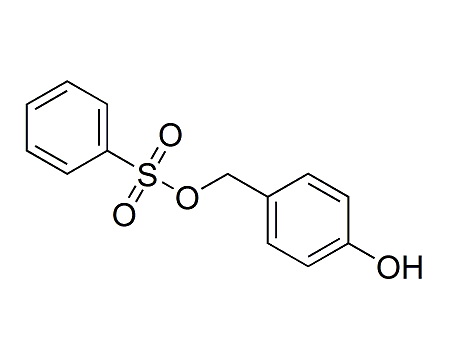
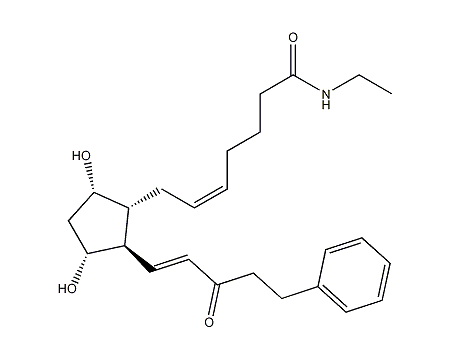
We are the leading manufacturer and supplier of pharmaceutical reference materials, such as drug working standard, drug impurity standards, drug metabolites and stable isotope labelled compounds. Our professional knowledge and service levels has made us one of the most trusted companies in this industry. We are the front runner in manufacturing complicated drug impurity standards or reference materials and we are the integrated solution provider in identifying, synthesizing and qualifying any unspecified impurityompound libraries. As a research-driven organization, we have developed novel processes to make niche products required to pharmaceutical companies globally at various platform. We understands the industry research needs thoroughly and has earned reputation for being most reliable innovator of pharmaceutical reference standards worldwide.
We have our own research lab equipped with advanced technology and smart team for organic synthesis and biosynthesis. Our laboratory is awarded ISO 9001:2015 Quality Management System certification. Our products are mainly used for R&D of pharmaceutical and bio-tech corporations, clinical and biological CROs, as well as universities, hospitals and other research institutions. Up to now, we have established long-term in-depth cooperation with many customers all over the worldwide.
We have profound experience in synthesizing complicated drug impurity standards or reference materials thus we are the comprehensive solution provider in identifying, synthesizing and qualifying any unspecified compounds. As a R&D oriented organization, we have developed novel processes to make our characteristic products available for global pharmaceutical companies at various platform.
We know that quality of product is quite important for clients. So we attach great importance to the stability and uniformity of all products and assure an uncompromising level of quality is offered for each reference standard provided. Every compound we synthesized is accompanied with a complete COA supported by comprehensive analytical data of MASS, 1H-NMR, HPLC, etc.. For some special standards, we can provide 13C-NMR, IR, H-H COSY and DEPT according to the requirement of per client and the specific characteristics of the concerned product.
Kindly be noted that Aozeal Certified Standards, Inc. (hereinafter referred to as AOZEAL) grant the authorization to SUNGENING MEDICAL(HONGKONG) CO.,LIMITED (hereinafter referred to as SUNGENING) for all sales and marketing related matters. Invoices and other documents will be issued by SUNGENING and letterhead in the future.
We fully understand our quick development over the past years derived from our valued customers’ trust and support. AOZEAL will consistently provide you all with more comprehensive and convenient products and services.
From general manager of Aozeal Certified Standards, Inc.
22th February 2023